
Cefalexin for UTIs – Right treatment Length in Young children
Eligibility
Local study conflicts
- Cannot enrol if patient is on any other drug trial
Inclusion Criteria: (all criteria must apply)
- Age 3 months to 11 years inclusive
- Clinical diagnosis of febrile UTI at presentation to ED as defined by BOTH:
- Temperature ≥38°C measured by any method or likely fever in the last 24 hours
- Clinical feature(s) suggestive of UTI at presentation – at least one symptom:
- Early urine test suggesting likely UTI as defined by any one of the following:
- Dipstick: nitrite positive AND leucocyte esterase positive.
- Dipstick: nitrite positive AND leucocyte esterase negative (on a fresh urine sample).
- Dipstick: leucocyte esterase positive AND nitrite negative AND the treating clinician assesses there to be good clinical evidence of a UTI (e.g. obvious urinary symptoms).
- Abnormal urine microscopy (bacteriuria by microscopy with Gram stain)
- Decision to treat with oral cefalexin
- Plan for discharge from the ED
- Able to give written informed consent and use the App
Exclusion Criteria:
- Known congenital anomalies of the kidney and urinary tract (CAKUT), reflux nephropathy or indwelling catheter
- Known immune deficiency or currently receiving immunosuppression therapy
- Systemic antibiotics for any reason (treatment or prophylaxis) in the previous 14 days
- Weight > 50kg
- Known allergy to cefalexin or previous severe allergic reaction to any beta-lactam antibiotic
- The patient is on any other drug trial
If the patient is eligible:
Step by Step Guide to Recruitment:
In standard working hours; 9-5
- Please contact the ED Research Team when eligible patients arrive in ED:
Send the ED Research Team a message via Epic chat "ED research Team" or phone 217907
Out of hours and weekends:
We are not planning to recruit OOH for the foreseeable future
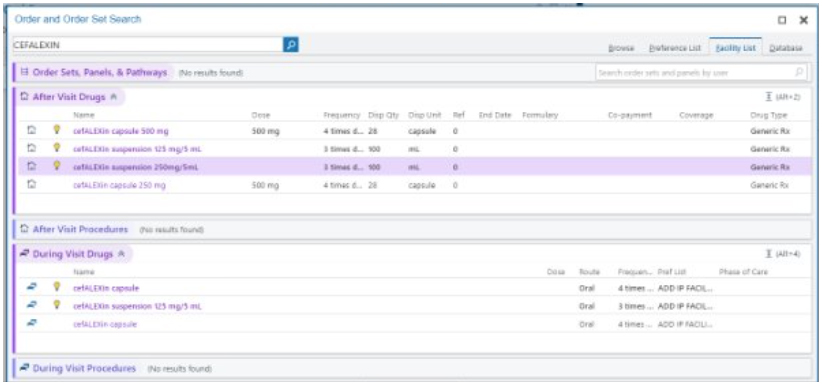
How to prescribe
For those on the delegation log:
Click here:
Links for further information:
Link to local trial site
Link to patient information leaflet
Tap the button below to display a QR code link to the patient information leaflet:
For local training to get on the delegation log, contact the ED Research Team to arrange access to the Birmingham TEAMs Channel
